WhiteGAPS Evaluation Results


© 2012 WhiteGAPS (All Rights Reserved)
An evaluation was conducted to ensure that WhiteGAPS are safe and effective barriers in guarding patient health by helping prevent cross-contamination through nitrous oxide hoods.
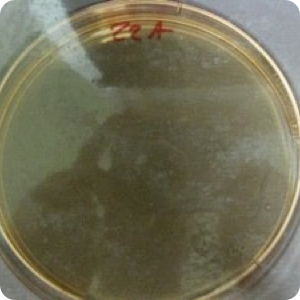
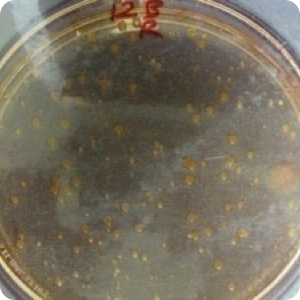
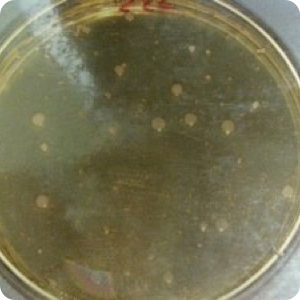
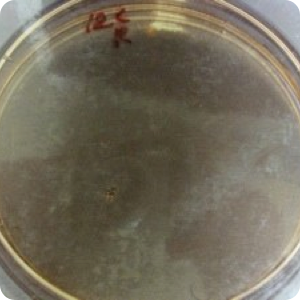
Results: Significant protection (P<.02) from cross-contamination was provided by the use of WhiteGAPS. No bacterial growth was found on any plates of samples taken from the hoods after surgery in which WhiteGAPS were used. However, bacterial colonies were seen in post-surgical samples from 25 of 30 non-WhiteGAPS patients. Pre-surgical samples from control patients showed four cases of growth; the WhiteGAPS group showed eight cases. These were determined to be incidental growth and not due to insufficient disinfection procedures. Mannitol salt agar was used as a screen for Staphylococcus aureus, so lack of growth on this medium indicated an absence of S. aureus. but not necessarily an absence of other bacteria. TSA was used to show the presence of other types of bacteria in general.
Image 1: Pre-surgical swab of hood
Image 2: Swab of Nasal Alae & Nares
Image 3: Post-surgical swab of hood without WhiteGAPS
Image 4: Post-surgical swab of hood with WhiteGAPS





